The ROS1der Story (my talk at the 2017 IASLC World Lung Cancer Conference in Japan)
This year I was honored to be invited to speak at the 18th IASLC World Conference on Lung Cancer in Japan. This is a huge annual conference, with about 7,000 attendees, which takes place in a different location every year. My talk was titled “The ROS1der Story: How a Group of Patients, Advocates and Doctors Launched an International Research Effort.” Below, I’ve included my slides and some of the remarks I made about each:
__
Slide #1:
I’m here to speak about the ROS1der story. We are breaking new ground, collecting information and biospecimens from patients across the globe and enabling research and development of treatments for ROS1+ cancer in ways that would never have been possible before.
__
Slide #2:
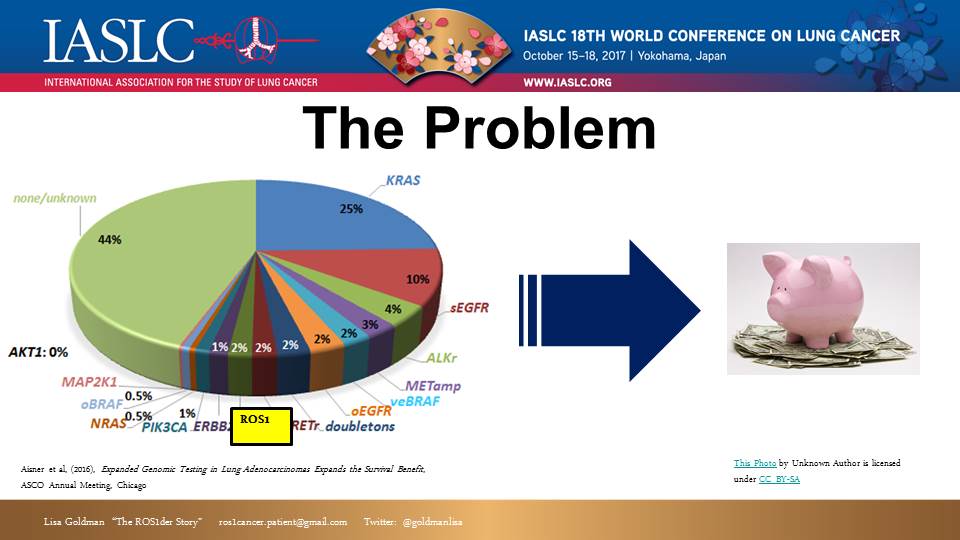
Back in 2015, I attended the IASLC WCLC in Denver, Colorado. At that time, I had been on a targeted therapy called Crizotinib for about a year. I knew I was approaching the median effective time for this treatment, and I was trying to prepare and figure out what treatment I might shift to next. I had coffee with Dr. Ross Camidge, a top ROS1 researcher, to discuss this. Dr. Camidge reported that there was very little on the horizon for ROS1+ patients. Why? Because researchers had a hard time collecting enough ROS1+ patients to conduct valid studies.
The problem came into clear focus: ROS1 fusions represent 2% of NSCLC patients. If every NSCLC patient were tested, about 2,000-5,000 patients would be identified each year in the U.S. (many thousands more if you add in other countries, and also the 9 other types of cancer where ROS1 has been found). That’s a small population within the greater cancer community. Small population size means difficulty gathering both a sufficient patient cohort, and sufficient research investment.
__
Slide #3:
This was a problem that I and other ROS1+ patients were very motivated to resolve. We developed a 5 step approach to instigate a greater investment and more research into ROS1+ cancer to prolong our lives.
__
Slide #4:
First: We formed a team. This was an informal, organic process. Patients and caregivers interested in spearheading this project met online and at conferences. There were not any eligibility requirements, but in hindsight, here are some characteristics of our leadership team that helped make it work:
- Diverse & Complementary Skill Set – Nobody had directly relevant medical research experience, but we did have people with backgrounds in business, science, law, writing, web design, marketing and project management.
- Time & Health – The demands of this project are significant (e.g. weekly calls, tasks, travel). We all committed and most of us had the good fortune to enjoy extended periods of good health to do this.
- Flexibility – We are not a dictatorship, nor are we leaderless. As a patient group, we cannot risk having just one leader or a very strict organizational structure. We have to accommodate the realities of living with advanced cancer.
- Scientific Capability – It turned out to be crucial to our project to have a few leaders that really understood the complex science and who could communicate both with the researchers and then back to the less scientifically sophisticated in our group.
- Disincentives – It’s important to note that having a group of patients and a thriving online community is not enough in and of itself to create a leadership team who will launch a program like this. There are many reasons people may choose not to do this, including: discomfort in working with others, feeling lacking in skills or authority, can’t afford the time/money/energy, don’t have a tolerance for some criticism, preference to work on shorter-term efforts with more immediate results than medical research which can take years.
__
Slide #5:
With a leadership team in place, we moved on to Step 2: gathering ROS1+ patients to facilitate research. We didn’t need to reach every ROS1+ patient, or even a majority. We succeed if we achieve a critical mass.
One big hitch was patient privacy. We couldn’t reach patients directly (unless they made themselves and their ROS1 status public), so we had to make ourselves as public as possible and help them find us.
__
Slide #6:
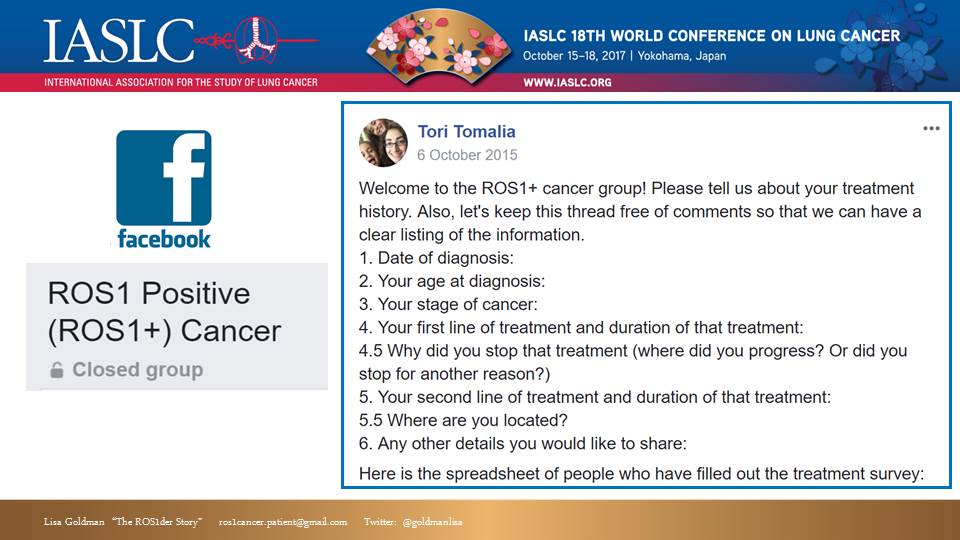
Our first and probably most effective tool for aggregating patients was our private ROS1+ Cancer Facebook group (which also happens to be a very useful community for ROS1+ support). [Side note: we also tried some other online platforms like Inspire and Smart Patients for our group, but FB clearly had the most momentum, so we focused on that.]
People can find us by searching FB for “ROS1,” but since it is a private group, they cannot see the content of the group unless they are accepted as a member.
__
Slide #7:
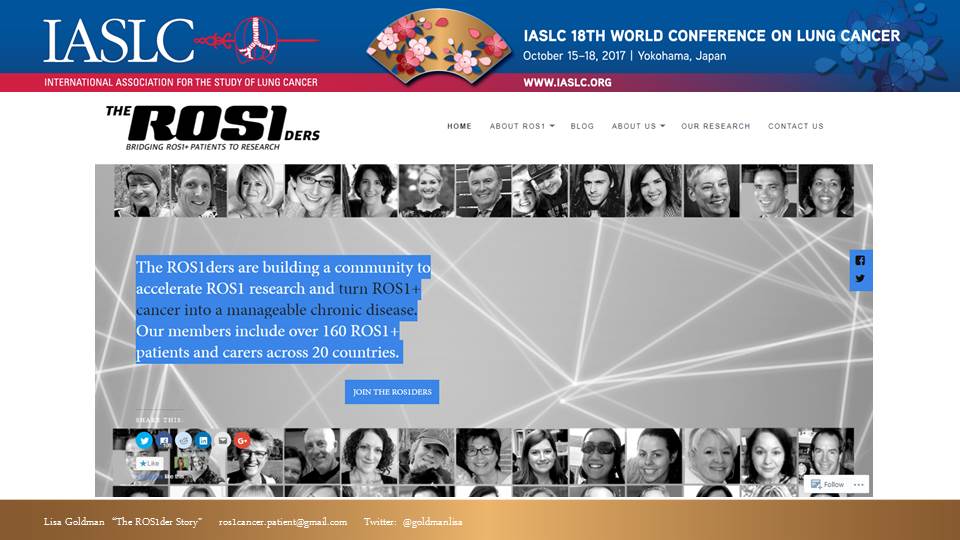
Later we recognized the need to also have a public-facing, non-private presence online. So we created a website: ROS1cancer.com. This public website helps raise our profile for other patients searching for us, and also offers a venue for non-patients interested in learning about or contacting us (e.g. doctors, researchers, journalists, advocates for other health concerns). It also lends our effort a more professional credibility.
__
Slide #8:
To help spread the word, we created flyers and passed them out to our doctors and oncology centers and distributed them at conferences. We have translated these flyers into several languages which can also be found and downloaded from our ROS1cancer.com website.
__
Slide #9:
In further efforts to reach and aggregate ROS1+ patients, we have: blogged, done press interviews (both mainstream and industry), attended and spoken at conferences, scoured the web for other patient mentions of ROS1, and even created a really awesome animated video.
__
Slide #10:
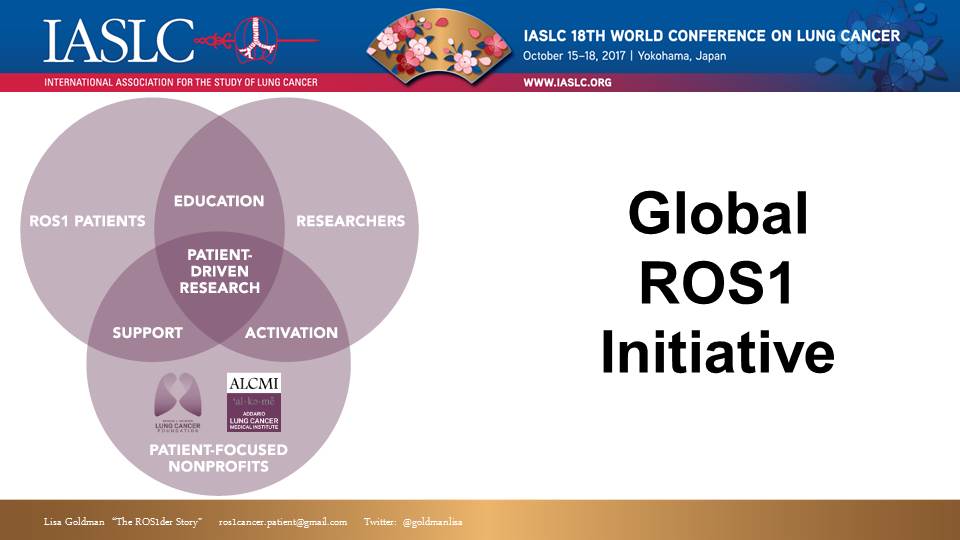
Step 3: Once we had aggregated a critical mass of ROS1 patients (about 50). We reached out for help. I called Bonnie Addario at ALCF. I knew that ALCF had experience launching research and thought they might be able to help. Luckily, the ALCF and their sister organization ALCMI embraced us. Their professional experience and support has been invaluable in many ways, including helping guide our next step…
__
Slide #11:
Step 4: It is critical to consider the needs of a group before launching any sort of major effort. With the help of ALCF and ALCMI, and researchers, we learned that in order to generate more and better treatments for ROS1+ cancer, we needed to create more cancer models (cell lines and mouse models). So, rather than focusing on any particular type of treatment (chemo, targeted therapy, immunotherapy, vaccination, combinations of these things, etc.), we focused on figuring out how to generate more ROS1 models in general, and how to make them widely available to as many researchers as possible.
It’s important to highlight the unique needs of the ROS1 group here. Typically, historically, when patients donate bio-specimens for research, the researchers (or their employers) then own that bio-specimen and keep it proprietary. In the case of ROS1 – we know we have a very limited pool of patient samples. So, we cannot afford to abide by these historical research norms. Our priority is to get as many cell lines and models out to as many researchers as possible. This requires us to upend some long held practices. As patients, we are changing the way things are traditionally done.
__
Slide #12:
Step 5: Together we leveraged a team-based approach to achieve our goal. The ROS1der effort is truly a partnership between patients, researchers and advocacy organizations. “Patient Driven” means we have an active role in the process – not just raising money, or serving as the “face” for marketing and publicity. We all contribute significant time, energy, resource and skills. This became the “Global ROS1 Initiative.”
__
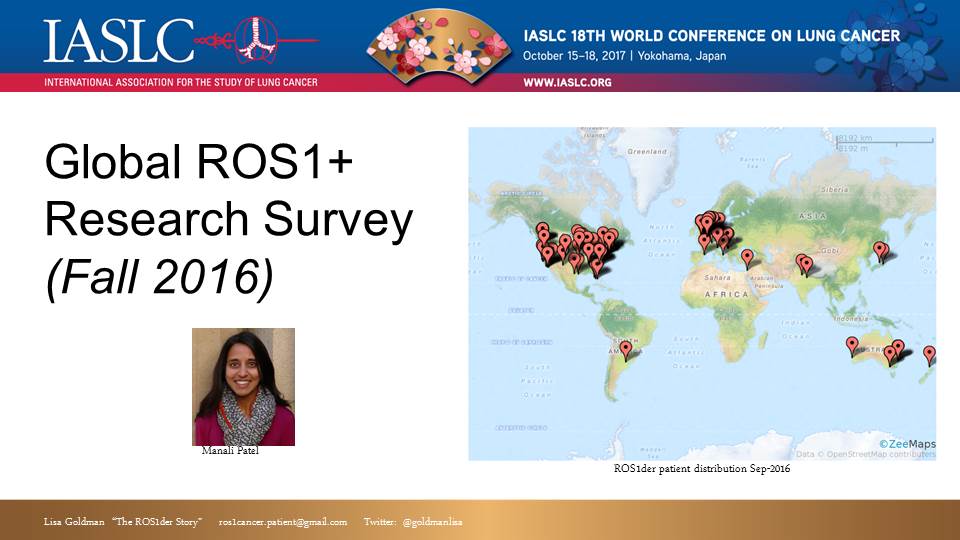
Slide #13:
The first project of the Global ROS1 Initiative was to conduct a survey, gathering more data about ROS1+ patients. We shared initial results of that survey at last year’s IASLC WCLC in Vienna. We have gathered more data since then and we are in the process of launching a second generation of that survey.
__
Slide #14:
After the survey, we shifted focus to collecting actual ROS1+ biospecimens for creating cell lines and mouse models for lab research. In August, we announced our partnership with ALCF, ALCMI and Champions Oncology with Dr. Christine Lovly as principal investigator. We are also in discussions to partner with Drs. Doebele and Shaw and exploring options with researchers in other countries as well.
__
Slide #15:
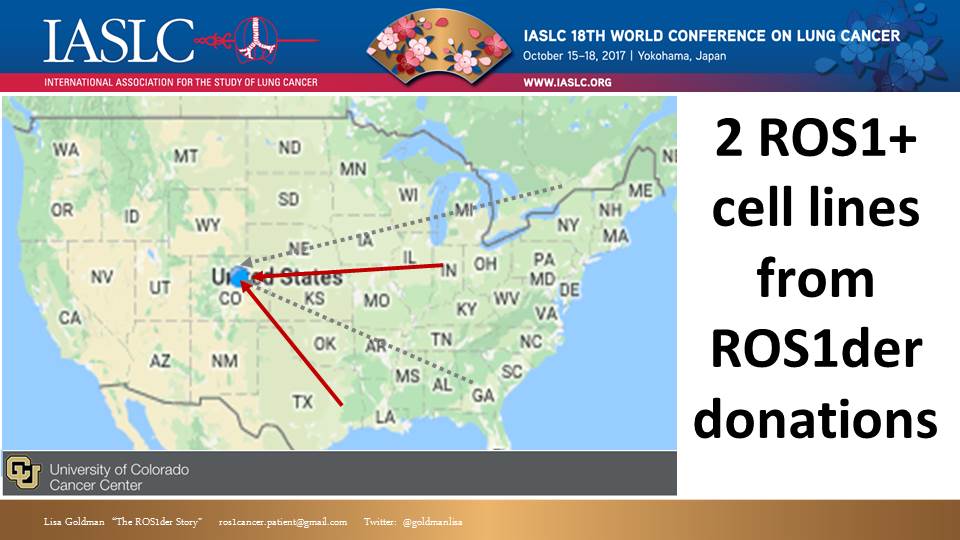
As proof of concept, I am happy (thrilled!) to report that prior to even formalizing partnerships, we have collaborated with University of Colorado. ROS1+ patients contacted the ROS1der team leaders in advance of a procedure and we contacted Dr. Doebele at Univ. Of Colorado. Dr. Doebele then coordinated directly with the patient for remote consent and arranged for express shipment of materials. The pleural effusion or tissue biopsy materials were received at Univ. of Colorado within 24 hours and two cell lines have been successfully created.
Two cell lines might not sound like a lot, but since we only knew of about 5 ROS1 cell lines in existence in the world previously, these additional 2 – before even our official launch – is significant and gives us great hope for the potential of this Initiative to really impact the future of research for ROS1+ cancer.
______________________________________________
This was my first trip to Japan (or anywhere in Asia). So, Eric and I took a couple of days to tour Japan a bit. We had a great time and particularly enjoyed the sights in Kyoto and Kamakura. Our photo album.